Flexible Ready-to-Customize Platforms
Fully-Programmable, Rechargeable Pulse-Generator, Controller, Wireless Charger and Wireless Powering Platform with Titanium, Ceramic and Peek Enclosure Options
01. FLEXIBLE MODULAR DESIGN
Every feature is modular: Simply remove the modules you do not need by not populating the hardware components... If you need a device with only 2 electrodes, 16 electrodes, or any number of electrodes in between, you do not need to change the design; simply do not populate the unused modules. The same applies to any other feature you may not need, including the near-field or far-field radio modules.
The design features a highly-efficient SCMR (Strongly Coupled Magnetic Resonance) wireless power transfer technology, that can be converted to the traditional Inductively Coupled wireless power transfer by simply removing the resonance coil assembly. Therefore, instead of using the RF-transparent ceramic can, one can use a traditional titanium case. However, if you so desire, SCMR can still be kept if the power transfer antenna is placed inside the silicone header!
IT IS A FULLY-PROGRAMMABLE AND TOTALLY-MODULAR DESIGN THAT CAN BE ADOPTED FOR PRACTICALLY ANY IMPLANTABLE MEDICAL APPLICATION.
We believe that "smart" devices will enable doctors to make more effective therapy choices for their patients. These devices will lower costs to the healthcare system, lower costs to the caregiver and keep patients in the therapeutic range of treatment more often. The Lone Star platform is fully programmable through simple C-code that can be adopted to any conceivable indication, including indications that have not as yet been discovered. This is the first software-defined indication platform in the world.
The design features a highly-efficient SCMR (Strongly Coupled Magnetic Resonance) wireless power transfer technology, that can be converted to the traditional Inductively Coupled wireless power transfer by simply removing the resonance coil assembly. Therefore, instead of using the RF-transparent ceramic can, one can use a traditional titanium case. However, if you so desire, SCMR can still be kept if the power transfer antenna is placed inside the silicone header!
IT IS A FULLY-PROGRAMMABLE AND TOTALLY-MODULAR DESIGN THAT CAN BE ADOPTED FOR PRACTICALLY ANY IMPLANTABLE MEDICAL APPLICATION.
We believe that "smart" devices will enable doctors to make more effective therapy choices for their patients. These devices will lower costs to the healthcare system, lower costs to the caregiver and keep patients in the therapeutic range of treatment more often. The Lone Star platform is fully programmable through simple C-code that can be adopted to any conceivable indication, including indications that have not as yet been discovered. This is the first software-defined indication platform in the world.
02. VERY LOW COST
- No ASIC: No Feature Lock-In, No Application Specific Integrated Circuits
- Very Low Material Cost via Off-the-Shelf Parts
- Very Low Manufacturing Cost; No Special Manufacturing Processes
- Mil-STD-883 Test Method 1014 Hermetic Titanium (6AL4V and 6AL4V Medical Grade) or Zirconia Ceramic Options
- Semi-Hermetic Enclosure to Accelerate Medical Research, Acute Studies, Animal Studies
- Modular Design; Populate and Activate Only the Required Modules
- Compelling Cost Reduction via Off-the-Shelf Parts as Manufacturing Volume Increases
03. UNIQUE FEATURES
- Wireless Upgrade Capability (after Implant Surgery)
- Independent Current and Voltage Sources
- Simultaneous Current and Voltage Stimulation
- Multi-Polar Stimulation Mono-/Bi-/Tri-Phasic; Anodal Block
- (Optional) multi-channel bio-potential monitoring capability with low-noise amplifiers
- (Optional) DC (Direct-Current) Capability
- Universal Pulse Protocols:
- Low/High Frequency (<1 kHz, >40 kHz) Tonic Pulses
- Burst Pulse Generation
- Simultaneous Low- and High-Frequency Pulses
- Any Waveform: Pulse, Sine and/or Arbitrary (signal envelope)
- Bi-Directional Near-Field Communications (up to 1.75 inches)
- Bi-Directional Far-Field Communications (MedRadio Band), Up to 15 Feet in Air
- Long-Life (typically 10 years) Rechargeable Battery
- External RF Powering (up to 1.75 inches)
- Simultaneous RF Wireless Powering and Battery Charging capability
- Battery and External-Temperature Monitoring
- Precision Battery-Charge and Battery-Health Monitoring
- Electronic Signature (Serial Number) for Authentication
- Emergency Shut Down via an External Magnet
04. ERGONOMIC DESIGN
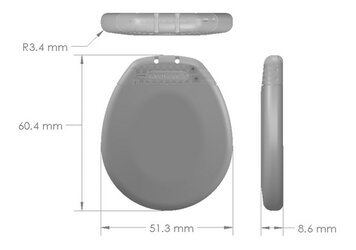
- 19.5 cc, 8.6mm Thick Can; One of the Smallest in the Industry (click on the picture to enlarge)
- Die-Scale, Off-the-Shelf Parts for Ultimate Miniaturization
- Modular Design; Unused Features Can Be Physically Removed
- Can Operate Without A Battery
- SCMR RF Wireless Power Feeding with Auto-Tuning
Click to View Wireless Power / Charger Auto-Tuning:
05. CLOUD READY
Medical-device companies have never had a better opportunity to be the bridge that connects healthcare providers to their patients. As hospitals and clinicians seek to deliver health services anywhere, medical-device companies can be key to bridging distance, time and boundaries between clinicians and patients thanks to the latest advances in telecommunications. Our platform can be made to communicate with the internet via simple Wi-Fi connectivity (or, optionally, via Bluetooth technology) by pairing with any mobile device and 3G, 4G or 5G mobile data path and connect patients with health-care providers in real time for two-way communication.
06. EASY ADOPTION TO EXISTING QUALITY MANAGEMENT SYSTEMS
LSNeuro platform is a programmable, indication-agnostic design that can be adopted for any external or implantable application and integrated into the specific quality management system of a medical device company. To facilitate this transformation, safety and efficacy aspects of possible targeted applications have been carefully considered through standards and agency guidelines, including but not limited to:
- FDA cGMP 21 CFR 820
- ISO 13485 rev 2016
- ISO 10993-1
- ISO 14708
- ISO 14971
- IEC 60601-1 Edition 3.1
- 47 CFR Part 15
- EN 62304
- EN 62366
- UL/IEC/EN 60950
- 93/42 / EEC, 90/385 / EEC
Copyright © 2014 - 2019 Lone Star Neuromodulation, Inc. All Rights Reserved.

